At the household level, corrosion is the appearance of rust on metal. But indeed, this is just an external manifestation. The most annoying point is what happens under the rust, i.e. the material destruction. The corrosion is defined as a spontaneous destruction of metals and alloys thereof during the chemical, electrochemical, or physical and chemical interaction with the environment. This article will consider only the electrochemical interaction, i.e. we will talk about the electrochemical corrosion.
The passage of the electrical current through the electrolyte is accompanied by ion movements. The positive ions go to the negative electrode (cathode), while the negative ions go to the positive electrode (anode).
Closed Galvanic Element
The most common example of the electrochemical corrosion is rusting of the metal parts in the moisture. In nature, no absolutely pure water exists, it always contains impurities that conduct the electricity. It means that a conventional, not distilled, water is always an electrolyte. In real-life iron, irregularities exist caused by the crystalline structure and different impurities. Due to this, the metal surface has zones with different electrochemical potential. The ingress of moisture to the surface of a conventional steel causes the formation of large number of closed microscopic galvanic elements. Currents flow therein and cause the electrochemical corrosion. Their values are insignificant, but during the long time period, serious destructions occur.
A more strong electrochemical corrosion appears when two items made of different materials are placed into the electrolyte. Then the potential difference between them reaches several volts, and the current value is also very large. Generally, a good galvanic element is formed. Such corrosion is observed in a cheap sanitary equipment of an unknown origin. After the 6 months of operation, the parts look like they have been in the concentrated acid solution. And the reason is in that the manufacturer, to provide savings, used in the taps or closing valves different metal with large difference in electrochemical potentials.
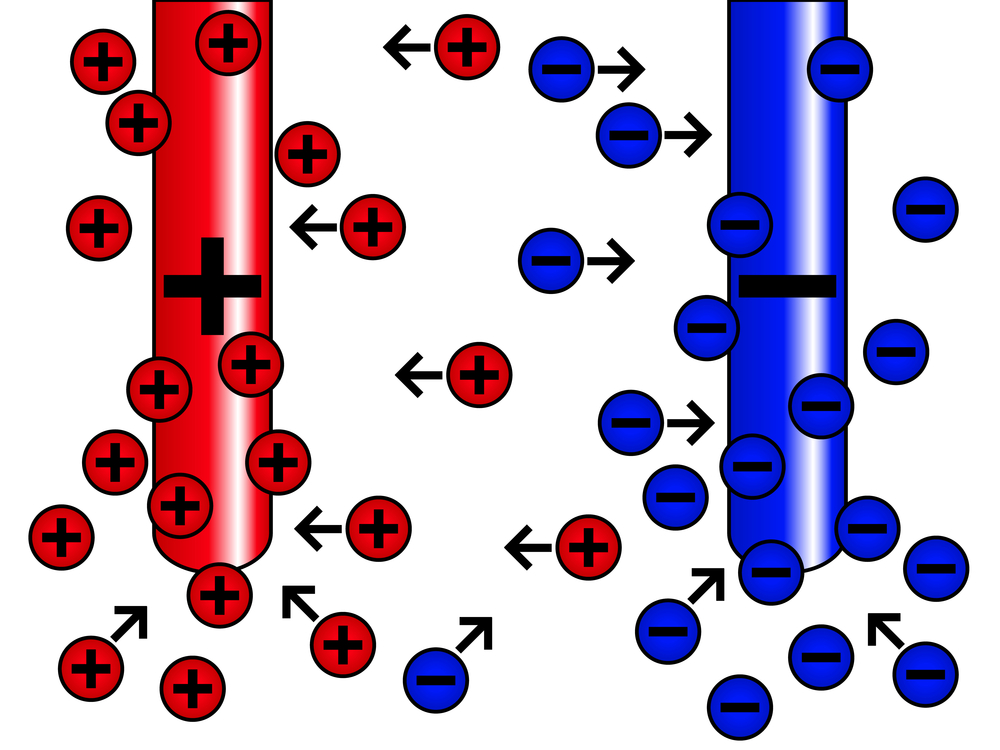
The electrical current in the electrolyte is associated with the transfer of ions of a substance, of which an anode is made.
The connection of the metal parts of the building and the earthing devices made of the zinc-coated steel with the basic potential equalizing system, the danger of the electrochemical corrosion occurs. Between the steel reinforcement of the reinforced concrete foundation and zinc, the potential difference occurs, which provides the corrosion of the earthing devices protective coating. The protective zinc coating will be dissolved thus reducing the steel reinforcement of the foundation.
Major Protection Methods
In order to struggle with the electrochemical corrosion caused by the formation of a galvanic pair, the following basic measures are applied:
- Using metal and alloys resistant to the corrosion. For example, copper or aluminum whereon a thin oxide film is formed which protects the items made of these metals from the corrosion. Or a stainless steel wherein the additives are added that change the internal structure of the material in such a way that it would not contain any irregularities with different electrochemical potentials.
- Galvanic coating. For example, a copper layer is applied to the conventional steel. Such protection is used in ready-made sets of ZANDZ earthing devices
- The coating made of a dielectric material resistant to the moisture, e.g. paint or lacquer.
- Use in moisture, is possible, homogeneous materials.
- Cathode protection that consists in the application of a negative potential to the protected element.
- Connection of zinc-coated earthing devices with the reinforcement in concrete only through the spark dischargers capable of conducting partial lighting discharges. The potential difference between the steel and zinc elements is much less than the discharger breakthrough voltage, therefore, in normal conditions, the electrochemical corrosion will not take place.
Cathode Protection
The cathode protection principle may be implemented in one of two methods. The first one is to supply the negative potential may be performed from a special power source called the cathode protection station.
In the course of the use of the cathode stations, the following problem may occur.
The electricity to install the required potential is often supplied along the long cable. This cable is exposed to the lightning action and some other electromagnetic impacts. In order to void the overvoltage as a result of this, it is recommended to use the integrated ZANDZ solution.
Conclusions
The electrochemical corrosion is a multi-aspect phenomenon. If you are not specialized in this subject during many years, it wil be very difficult for you to study why parts located in the water or in the soil degrade quickly. That is why in the aspects of protection against the electrochemical corrosion, especially when it may be caused by earth currents, it is better to rely upon the respected professionals. When you contact the ZANDZ.COM Technical Center, you may obtain a good consultation, the diagnostics of possible corrosion causes, and the ready-made solutions to protect against it.
Related Articles:
 ZZ-000-115 stainless steel grounding kit
ZZ-000-115 stainless steel grounding kit

 Grounding in the cellar of the single-family house - is it possible?
Grounding in the cellar of the single-family house - is it possible?
 Is Lightning Dangerous in a Large City?
Is Lightning Dangerous in a Large City?
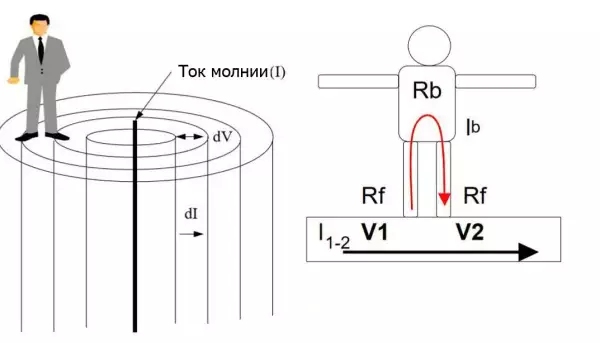 Step Voltage: Dangerous Obscurity and Reliable Protection
Step Voltage: Dangerous Obscurity and Reliable Protection
 Public Safety in Land Transport in case of Direct Lightning Strike
Public Safety in Land Transport in case of Direct Lightning Strike